Description
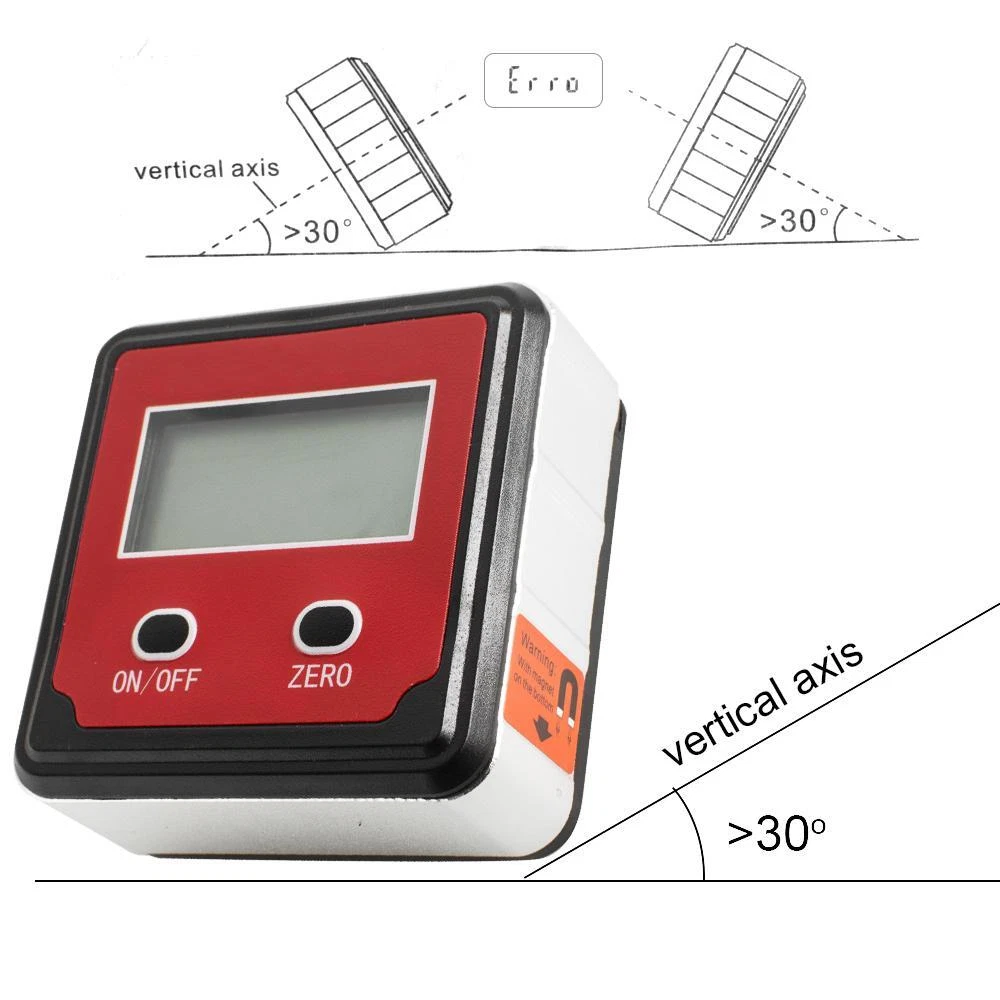
Description: Introducing the DUAL FUNCTIONALITY Digital Angle Gauge and Electronic Inclinometer, your essential tool for measuring and setting relative angles between surfaces. Featuring a large, easy-to-read display that remains on during use for enhanced visibility, this tool is equipped with a cutting-edge NEW MEMS SENSOR for exceptional accuracy and extended battery life. Ideal for tight spots, it displays bevels in degrees, IN/FT, mm/M, and percent slope. The STRONG MAGNETIC BASE securely attaches to metallic surfaces, while the reversible display ensures convenience even in awkward positions. With an IP54 rating for dust and water resistance, this gauge comes with two-lasting AAA batteries, auto shut-off for energy conservation, and a protective case with a belt for easy transport. Upgrade your measuring game today! Packing List: 1 x Digital Angle Gauge Package Including 1 x Digital Angle Gauge FDA Disclaimer: Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item. This item has been cleaned and treated according to the manufacturer's instructions.Ming-China-Beijing-86-19231142939 The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606. The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989 The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353 The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973 massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892 Ultrasound ,Ultrasonic Treatment Device is certified with the US FDA 510(k) Number:K161892 This item has been cleaned and treated according to the manufacturer's instructions.