Description
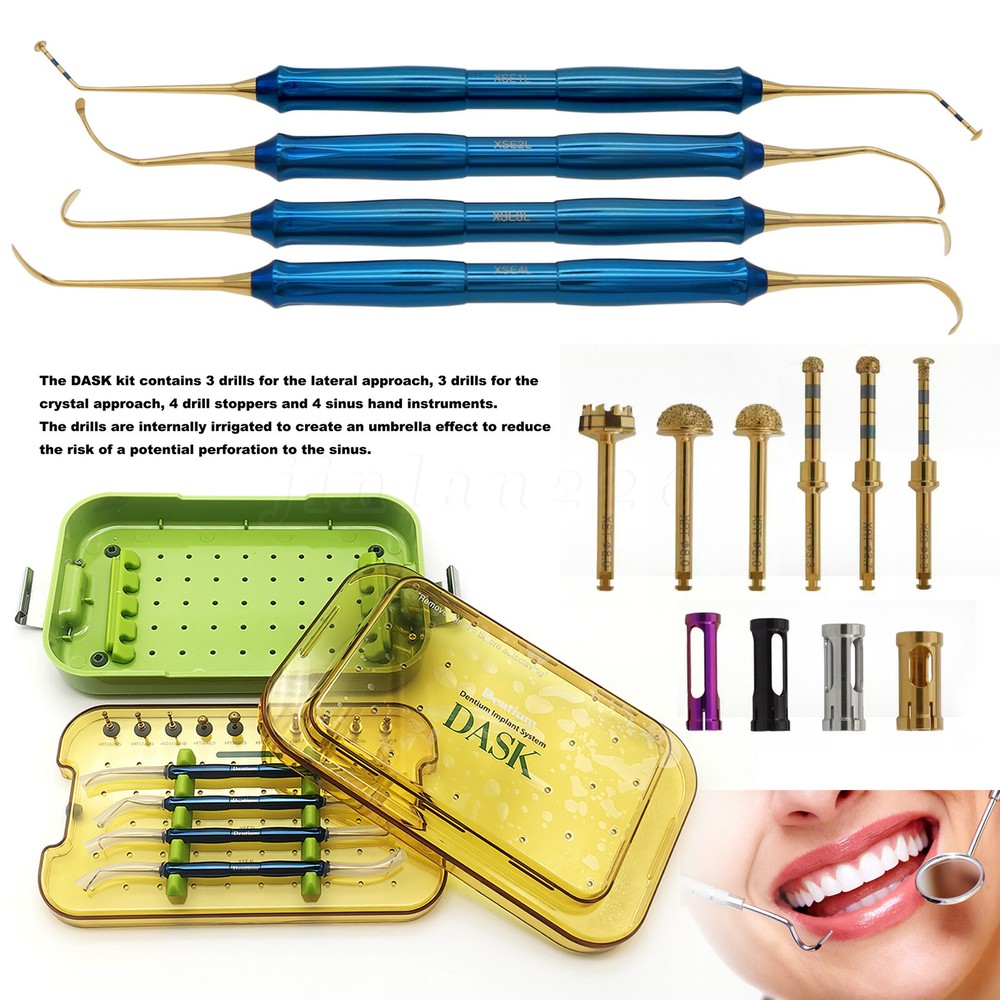
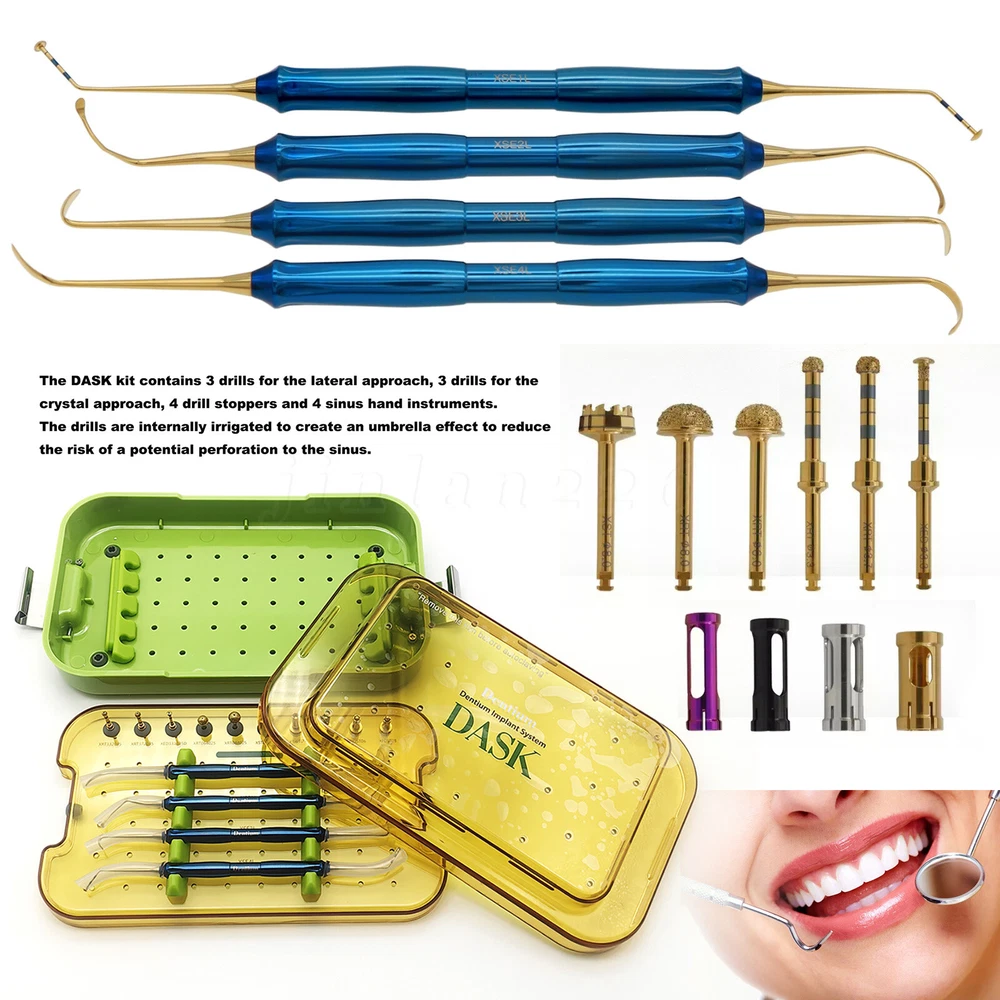
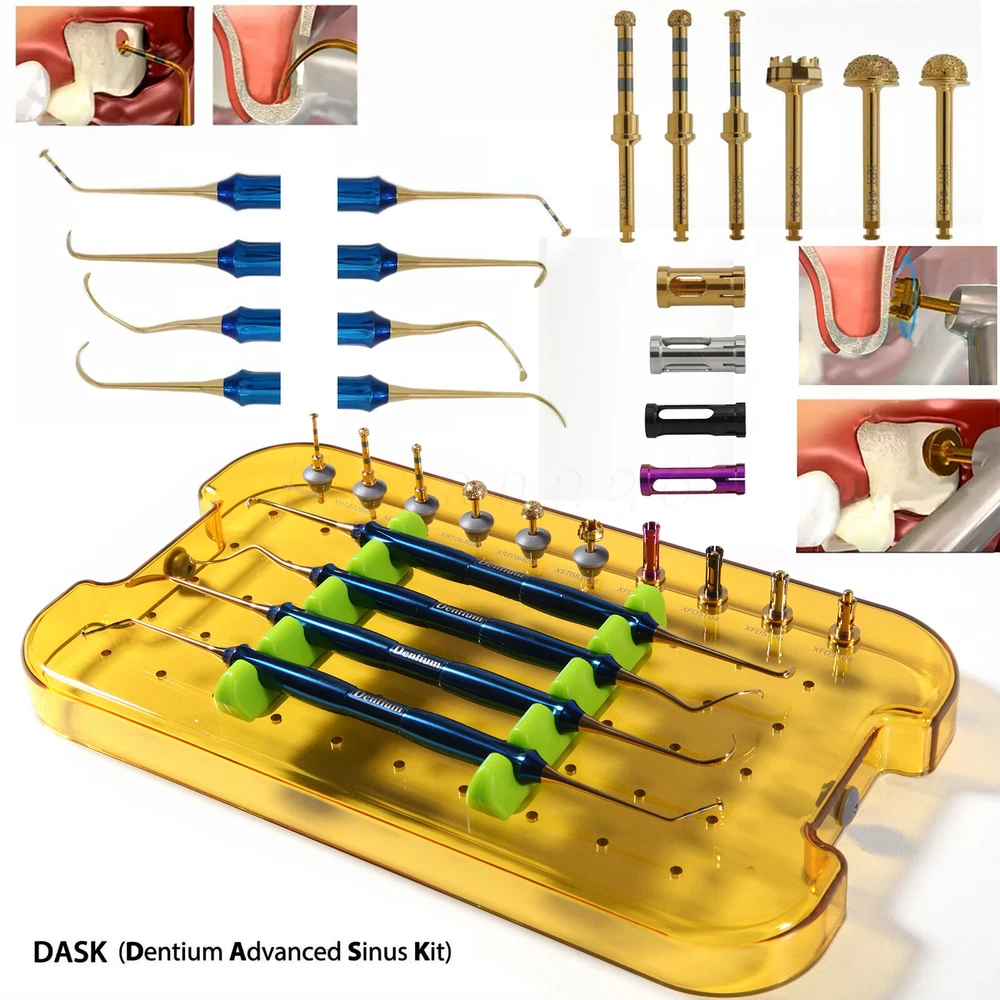
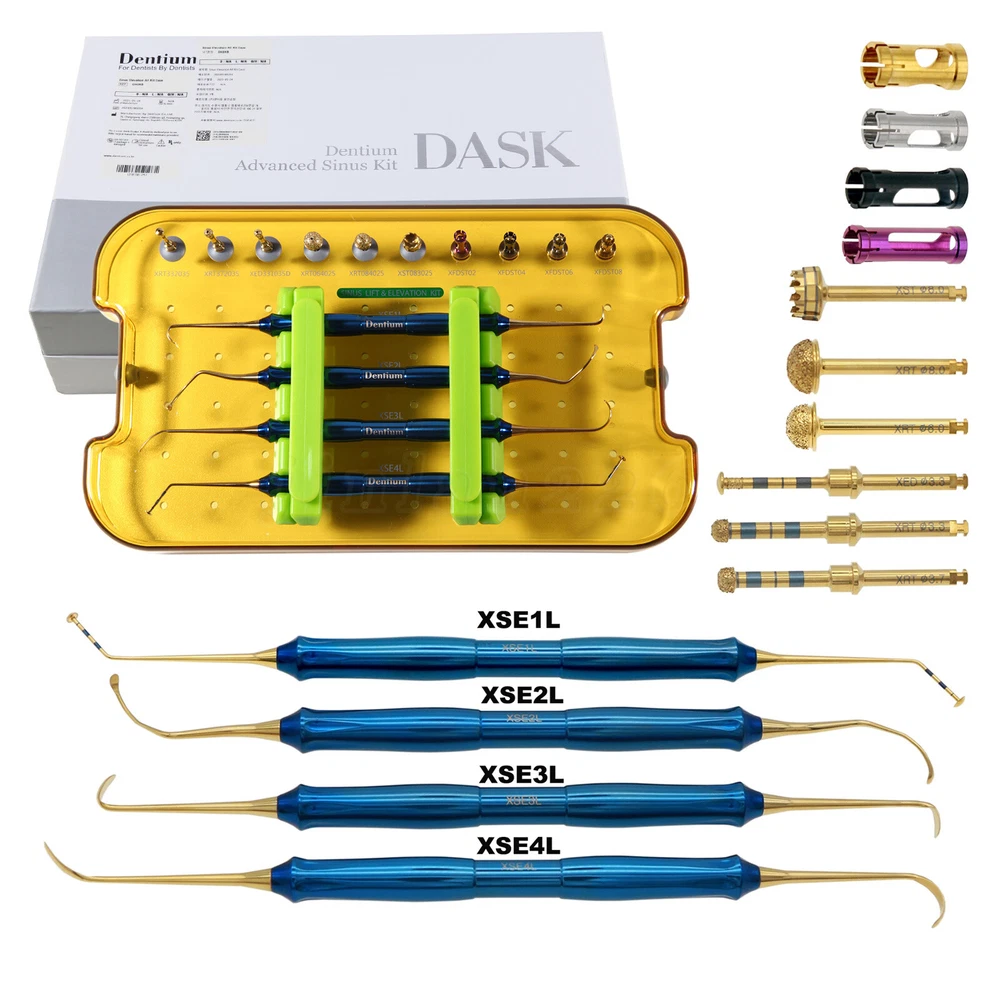
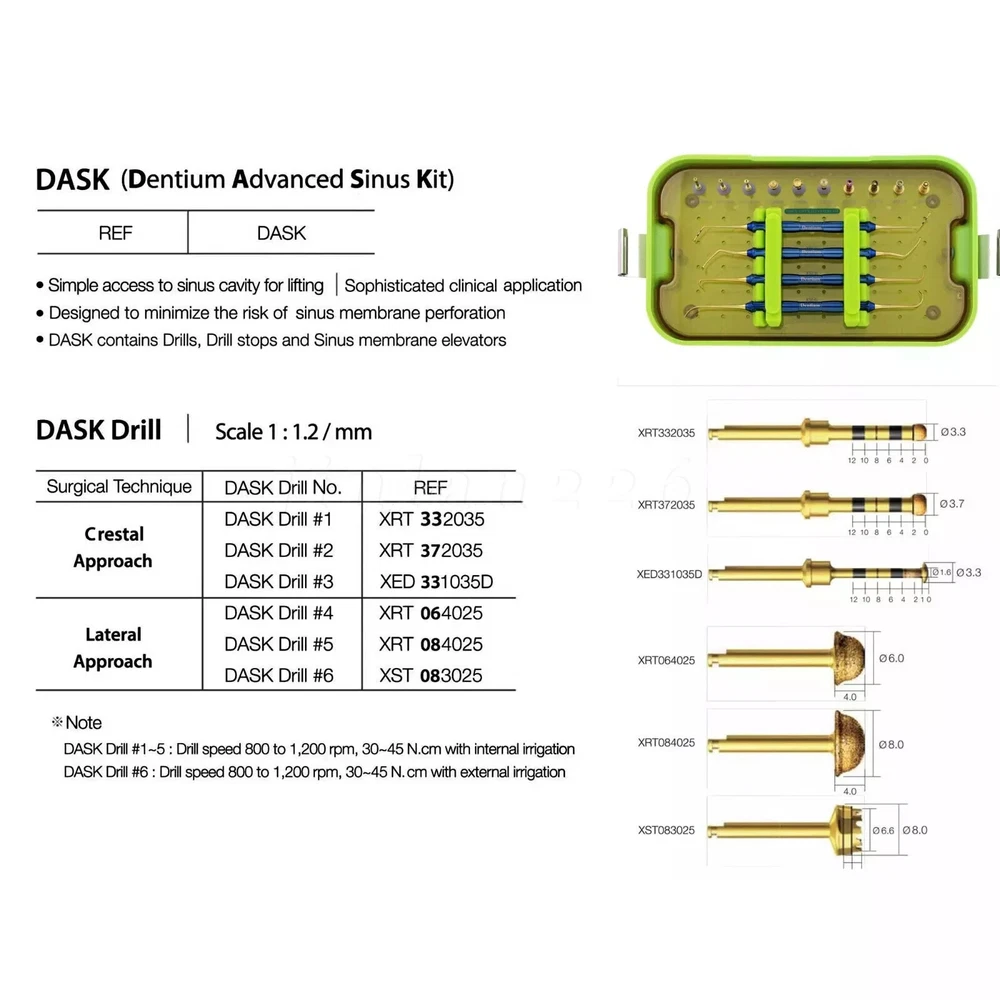
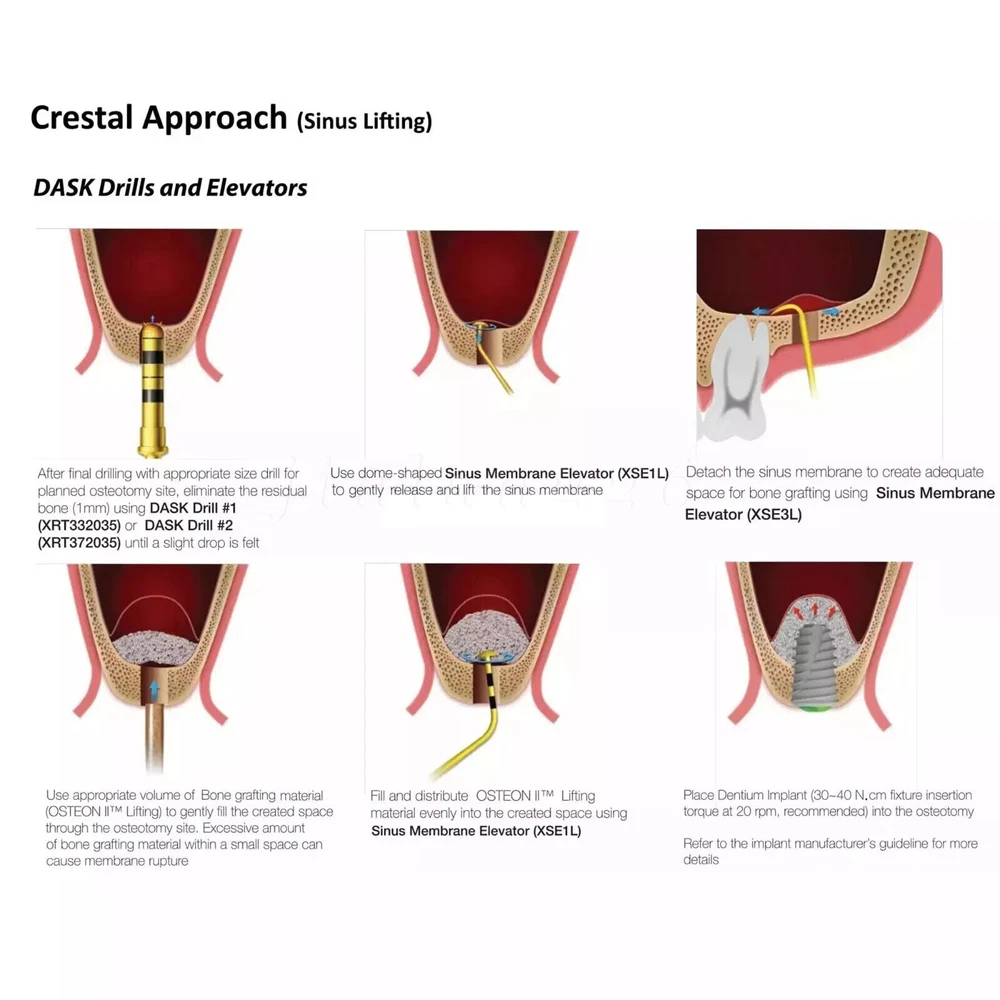
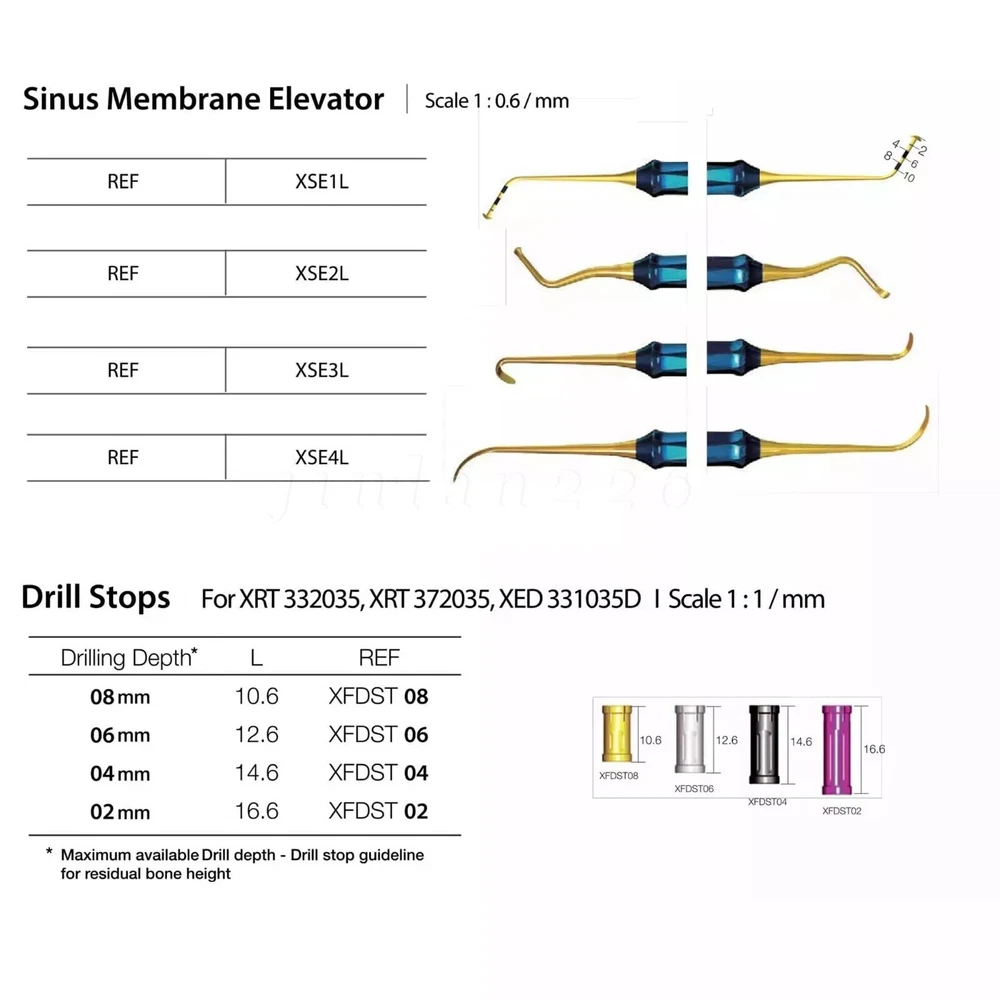
Description FDA for Handpiece: 510(K) Number: K181691 Regulation Number: 872.4200 Classification Product Code: EFB Subsequent Product Code: EGS Simple & easy access to sinus cavity • Broad exposure of bony walls with special instruments DASK is an advanced sinus lifting kit. The drills are internally irrigated, designed to create hydraulic pressure that protects the sinus membrane when drilling. The DASK kit contains 3 drills for the lateral approach, 3 drills for the crystal approach, 4 drill stoppers and 4 sinus hand instruments. The drills are internally irrigated to create an umbrella effect to reduce the risk of a potential perforation to the sinus. DASK Drill #1 XRT 332035 Crestal Approac DASK Drill #2 XRT 372035 DASK Drill #3 XED 331035D DASK Drill #4 XRT 064025 Lateral Approach DASK Drill #5 XRT 084025 DASK Drill #6 XST 083025 * DASK Drill #1~5 : Drill speed 800 to 1,200 rpm, 30~45Ncm with internal irrigation DASK Drill #6 : Drill speed 800 to 1,200 rpm, 30~45Ncm with external irrigation Stopper For XRT332035, XRT372035, XED331035D Drilling DepthLArt.No. 08 10.6 XFDST 08 06 12.6 XFDST 06 06 14.6 XFDST 04 02 16.6 XFDST 02 Sinus Elevation Instrument Art. NO XSE1L XSE2L XSE3L XSE4L FDA Statement: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, you can bid on this item only if you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item. FDA for Curing Light: 510(K) Number: K192233, Regulation Number: 21 CFR 872.6070, Product Code: EBZ FDA for Handpiece: 510(K) Number: K181691, Regulation Number: 872.4200, Classification Product Code: EFB, Subsequent Product Code: EGS FDA for Scaler: 510(K) Number: K163414, Regulation Number: 872.4850, Product Code: ELC Feedback: We commited ourselves to your 100% satisfaction both with our goods and services. 1. Please leave me FIVE star POSITIVE feedback if you are satisfied with our items. 2. Please CONTACT me immediately before leave us neutral feedback or negtive feedback, We will try our best to resolve any problem for you untill you are satisfied. Notice: 1. Import duties, taxes and charges are not included in the item price or shipping charges. These charges are the buyer’s responsibility. 2. Please check your country’s customs office to determine what these additional costs will be prior to bidding/buying. Warmly remind: Pls let me know before taking any action, because your action will has an important impact on our shop. So pls give me a chance to offer the best sollution for you!! Our working time: Monday - Friday 9:00 AM - 5:30PM (Beijing) Company name: Dreamland Technology Ltd City, State: Beijing, China Telephone number: 0371-56645662 Return & Refund: Return within 30 days is accepted, though the item in good condition. Copyright © jinlan226 On Sep 11, 2024 at 03:33:15 PDT, seller added the following information: On Jan 6, 2025 at 06:35:20 PST, seller added the following information: